Hall of fame
6RQA Crystal structure of the iminosuccinate ...

POLYVALAN is a privately held company specialized in the development, manufacturing and commercialization of innovative chemical tools dedicated to macromolecular biology. POLYVALAN is the exclusive licensee of a patent family owned by :
Ecole Normale Supérieure de Lyon (ENS Lyon),
Centre National de la Recherche Scientifique (CNRS),
Université Claude Bernard Lyon I (UCBL) and
Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA).
The patent application is covering both Tb-Xo4 products family and their applications in structural biology.
The Crystallophore/Tb-Xo4 inventors are :
Olivier Maury, expert of coordination chemistry and photophysical characterizations
François Riobé, organic chemist, expert in the field of supramolecular chemistry and functional materials
Eric Girard, biophysicist, he has been working for 20 years on the use of lanthanide complexes for biocrystallography
Sylvain Engilberge, biocrystallographer studying nucleating and phasing properties of Crystallophore/Tb-Xo4
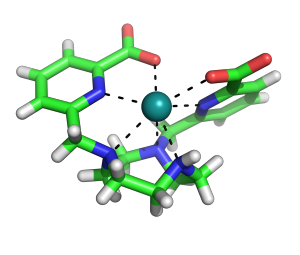
Polyvalan first product is a unique patented tool for protein crystallography that can solve three problems at once : forming, finding and phasing.
Crystallophore n°1 is a luminescent Terbium complex that is an effective nucleant and provides significant isomorphous and anomalous phasing power.
Tb-Xo4 should be dissolved by mixing it with 100μl of protein solution to reach a final Tb-Xo4 concentration of 10 mM. Then the crystallization experiments should be set up by mixing a volume of this « protein + Tb-Xo4 solution » with an equal volume of precipitant solution.
The proposed 10 mg/ml concentration is the optimal one. When hit is obtained, it’s possible to conduct an optimization by playing with the protein solution volume used in order to vary the Tb-Xo4 concentration from 2.5 to 15 mM.
There should be some competition with EDTA or EGTA to chelate the Terbium but we don’t know what will be the cinetic for a potential Terbium decorporation that could affect Tb-Xo4 efficiency. If EDTA or EGTA concentration is low (< 2 mM), this could only affect up to 20% of our Tb-Xo4 complex : the unaffected 8 mM Tb-Xo4 are then still falling inside its efficiency range.
Tb-Xo4 has been designed in order to be stable and highly soluble in aqueous medium and consequently fully compatible with high throughput screening. It is stable in all crystallisation conditions that have been tested ; please note that in some cases the presence of phosphate can be detrimental to the efficiency of Tb-Xo4.

There is direct interaction between protein negatively charged residues but not only.
It has never been observed but we cannot exclude it for very specific proteins.